Exothermic Welding Process
Amiable Impex is the Manufacturer of Exothermic Welding Powder, Exothermic Graphite Mold and Exothermic Weld Accessories.

 Exothermic weld process for joining two electrical conductors that employs superheated copper alloy to permanently join the conductors. The process employs an exothermic reaction of a copper thermite composition to heat the copper, and requires no external source of heat or current. The chemical reaction that produces the heat is an aluminothermic reaction between aluminum powder and a metal oxide.
Exothermic weld process for joining two electrical conductors that employs superheated copper alloy to permanently join the conductors. The process employs an exothermic reaction of a copper thermite composition to heat the copper, and requires no external source of heat or current. The chemical reaction that produces the heat is an aluminothermic reaction between aluminum powder and a metal oxide.
The reaction reaches very high temperatures, depending on the metal oxide used. The reactants are usually supplied in the form of powders, with the reaction triggered using a spark from a flint lighter. The activation energy for this reaction is very high however, and initiation requires either the use of a “booster” material such as powdered magnesium metal or a very hot flame source. The aluminum oxide slag that it produces is discarded.
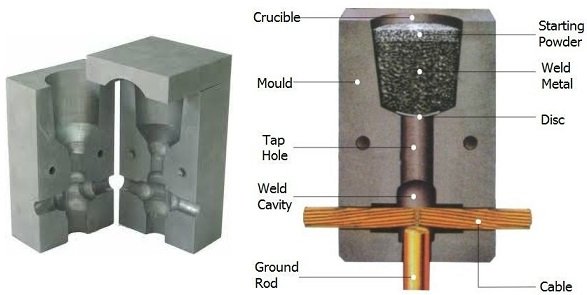
When welding copper conductors, the process employs a semi-permanent graphite crucible mould, in which the molten copper, produced by the reaction, flows through the mould and over and around the conductors to be welded, forming anelectrically conductive weld between them. When the copper cools, the mould is either broken off or left in place. Alternatively, hand-held graphite crucibles can be used. The advantages of these crucibles include portability, lower cost and flexibility, especially in field applications.
The weld formed has higher mechanical strength than other forms of weld, and excellent corrosion resistance. It is also highly stable when subject to repeated short-circuit pulses, and does not suffer from increased electrical resistance over the lifetime of the installation. However, the process is costly relative to other welding processes, requires a supply of replaceable moulds, suffers from a lack of repeatability, and can be impeded by wet conditions or bad weather.
Exothermic weld is a joining technique used to create a permanent connection between two metallic components. It involves a chemical reaction that generates heat, known as an exothermic reaction. This process is particularly noted for the durability of the bond produced and for preserving good electrical conductivity between the joined components.
Creating a bond by exothermic weld typically involves heat created by a chemical reaction between some type of heavy metal oxide and a reducing agent. For example, iron oxide is a commonly used metal oxide and aluminum is a common reducing agent. These reactants produce heat extremely rapidly when ignited, thereby achieving the high temperatures needed for welding.
Such heating is generally initiated once the parts to be joined are fitted together in a mold which contains the materials and the reaction as it takes place. Filler metal in liquid form is produced by this reaction and mixes with melted metal from the parts being joined to form a bond shaped by the mold. Molds used in exothermic weld may be made of graphite, ceramic, or other appropriate materials.
Exothermic weld connects cables, ground rods, terminals and structures; the resulting molecular bond produces a permanent connection that won’t loosen or corrode over the lifetime of the installation. You should use exothermically welded connections when: you require long life/permanent connections; you expect high current faults; or corrosive conditions exist.
The equipment required to make exothermic connections is lightweight, portable and does not require outside power. The resulting bond is a permanent joining of metallic parts that form an electrically conductive path. This will ensure electrical continuity and the capacity to conduct any potential current safely.
These permanent connections will carry as much, or more, current as the conductor. These connections eliminate bimetallic corrosion at the point of the weld. All strands of the conductor will equally share the current load.
Exothermic weld system from provides precision engineered molds for optimal results. Permanent molecular bonding is the preferred method for making copper to copper and copper to steel electrical connections. It is a connection using a high-temperature exothermic reaction that does not require an external heat or power source. Its performance is superior to all existing surface to surface connectors. Features include simplicity, corrosion resistance, extended shelf life, high conductivity, portability, ease of use, and it requires no external heat or power source.


Please Note – If there is even a minor gap between the two Conductors, apply Sealing Compound at the places where the conductors is passing out else at the time of Welding, the Powder will spread out as a flame resulting in improper joint.